CONTINUITY OF STATE
- At the critical temperature, the gaseous \(C{O}_2\) cannot be distinguished from liquid carbon dioxide which indicates that the conversion of \(C{O}_2\) gas into liquid \(C{O}_2\) or vice versa is not a sharp or discontinuous process but is a continuous process.
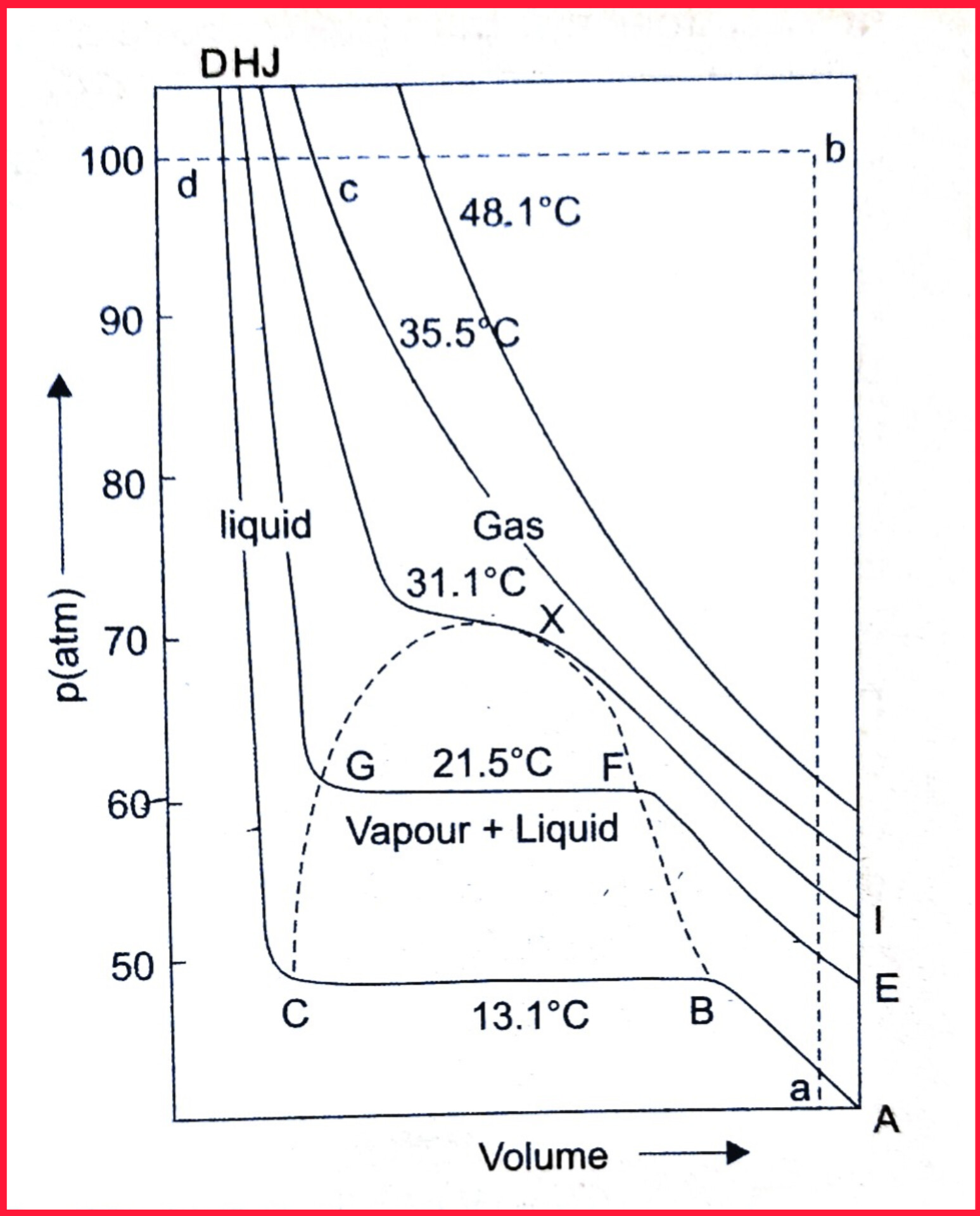
- As shown in the figure, if the ends of the horizontal portions of isotherms are joined together, a parabolic curve CGXFB represented by dotted line is obtained.
- The point X at the peak of the parabola represent, the critical point (31.1°C).
Within this parabolic curve, both liquid and gasoeus states coexist. - but outside the curve CGXFB
on the right side, only gaseous state (gaseous \(C{O}_2\) ) is present while on the left side only liquid state ( liquid \(C{O}_2\) ) is present. - Below the critical temperature, it is possible to change \(C{O}_2\) gas to liquid \(C{O}_2\).
• In other words, the change from gaseous to a liquid state can be carried out without any sharp discontinuity :
Consider the isotherms of carbon dioxide,
- Without having both phases together at any stage (e.g., at 13.1°C) if the pressure and volume corresponds to the point a, \(C{O}_2\) exists as gas.
- At point a , keeping the volume constant if gaseous \(C{O}_2\) is heated till its temperature rises above critical temperature and pressure reaches to point b. During this isochoric process, the pressure rises along path ab.
- Now If the pressure is kept constant and the gaseous \(C{O}_2\) is cooled so that the volume decreases from b to c.
- At point c, the intermolecular forces are large enough to cause condensation, hence at point c, liquid \(C{O}_2\) is obtained.
Thus along the path abc, there is only one phase present.
Thus the change in state from gaseous to liquid state or vice versa is continuous. This is called as the continuity of state.
Note:
At point a, \(C{O}_2\) is entirely gaseous, but at point c, it is completely in liquid state.
The change of state has occurred during compression along b to c but nowhere along the path a b c, the two phases coexist.(It is either gaseous or liquid state)

